This project is part of the EDCTP3 programme supported by the European Union




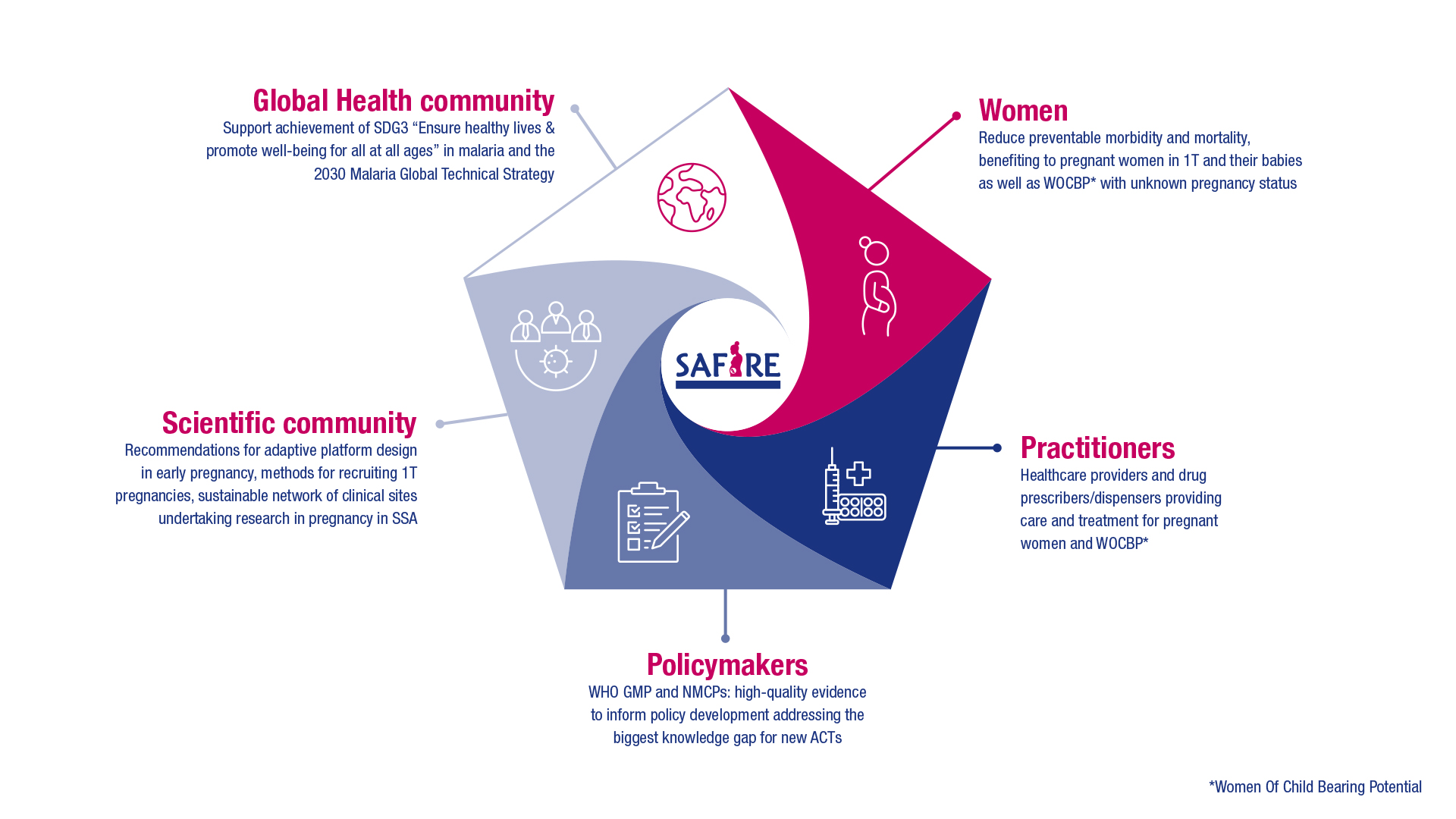
How will the successful dissemination and exploitation of SAFIRE project results impact the target group?
What are the expected wider scientific, economic and societal outcomes of the project?
Major milestone in Africa’s first-ever study assessing malaria treatments in early pregnancy Through the SAFIRE consortium, experts from Africa and Europe are closing a critical treatment gap for women in early pregnancy. Each year, over 12 million pregnant women are
Speakers and presentations Shaping the future: advancing equitable and ethical research to meet women’s therapeutic needs Location: Kigali Convention Center, Room MH3Date & time: Tuesday, 17 June from 16:30 to 18:00 Dr Stephanie Dellicour, Principal Research Associate, Liverpool School of
In a world first, a global consortium plans to undertake a Phase 3 clinical trial assessing antimalarial medicines in women in their first trimester of pregnancy The trial will evaluate the efficacy, safety, tolerability and cost-effectiveness of antimalarial drugs to
Malaria is a dangerous disease. One that, according to the World Health Organization, resulted in 249 million cases and 608,000 deaths in 2022 worldwide. For pregnant women, particularly those in their first trimester, it can cause serious complications, a concern for over 120 million women
Malaria is one of the most serious infectious diseases affecting predominantly low- and middle-income countries, where pregnant women are among the populations at risk. There are limited options to prevent or treat malaria in pregnancy, particularly in the first trimester,